The Secret to Naming Organic Compounds in Less Than Ten Minutes
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: alkanes
Alkanes are simplest hydrocarbons with all single bonds present within them and comprised of carbons and hydrogens mainly.
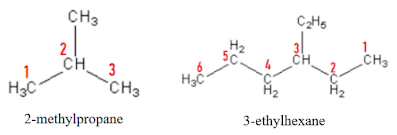
Logic pole: Ask yourself “How is the structure of the molecule, is it having an unbranched backbone or branched backbone?”
If it is having unbranched backbone
 |
| unbranched substance |
Use a prefix indicating the number of carbon atoms present in the chain and end its name with ‘ane’.
 |
| propane |
If it is having branched backbone
 |
| branched substance |
Count from each end for the number of carbon atoms in each possible chain and get the longest chain. The root name belongs to the longest chain or the parent chain. Start numbering each carbon in the parent chain from either side. The branched chains are now called as substituent.
Logic pole: Ask yourself “Counting from which end gives least number to the very first encountered substituent in the parent chain?”
Consider the least numbered counting for the first encountered substituent.
(i)
Check
the alphabetical order for the naming initials of the substituents, if more
than one substituent is present (-ethyl would precede -methyl). If you are
wondering about why ‘yl’ is used here instead of ‘ane’ with the carbon number
indicating prefix for the substituents, check here.
(ii) Tell the position of the substituent with numbers (2, 3 or whatever is applicable)
(iii) Place hyphen after that positioned number (2- .., 3-…)
(iv) If the same substituent is present in the compound for multiple times, use -di, -tri etc. after mentioning each of the positions with comma (2, 3, 4- tri……). Note that the -di, -tri are not included in alphabetizing.
(v) Finally, end the naming with the naming for the backbone of the parent chain.
There may be situation when counting from multiple ends of the branched alkane ends up with same chain lengths. In that case, choose that one as parent chain which is having a greater number of substituents in it.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
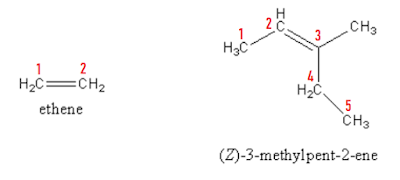
Naming Organic Compounds: alkenes and alkynes
Alkenes and alkynes are hydrocarbons having multiple bonds present in them. Alkenes contain double bond and alkynes contain triple bonds as the functional groups.
Follow all the steps as mentioned for naming the alkanes and give the least numbering position to the corresponding functional group. End the name with ‘ene’ and ‘yne’ for alkene and alkyne respectively.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: alcohols
Organic compounds with functional group -OH are named as alcohols. Follow the steps as mentioned for naming alkanes placing -OH in the least numbered position on the parent chain. Change the suffix of the corresponding alkane’s ‘e’ to ‘ol’. Remember that the location of -OH precedes to the location of any alkyl or halogen atoms while numbering in the parent chain.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: aldehydes and ketones
Aldehydes and ketones are organic compounds having -C=O as their functional groups. Aldehydes are having one alkyl group and one H on both sides of the -C=O group, whereas, ketones are having two alkyl groups on both sides of the carbonyl group. Follow the steps as mentioned for naming alkanes placing -C=O in the least numbered position on the parent chain. Change the suffix of the corresponding alkane’s ‘e’ to ‘al’ for aldehyde and ‘e’ to ‘one’ for ketone. For unsaturated aldehyde or ketone, molecule containing a carbon-carbon double bond or a carbon-carbon triple bond, infix ‘-en-’ and ‘-yn-’ should be used respectively.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: carboxylic acids
Organic compounds having –(C=O)-OH or -COOH as their functional groups belong to the class of carboxylic acids. Follow the steps as mentioned for naming alkanes placing -COOH in the least numbered position on the parent chain. Change the suffix of the corresponding alkane’s ‘e’ to ‘oic acid’ for carboxylic acid. For unsaturated carboxylic acid, molecule containing a carbon-carbon double bond or a carbon-carbon triple bond, infix ‘-en-’ and ‘-yn-’ should be used respectively.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: carboxylic esters
Carboxylic esters are organic compounds having R–(C=O)-OR’ or -RCOOR’ as their functional groups, where R and R’ are alkyl groups. The name of carboxylic ester starts with the alkyl or aryl groups bonded to oxygen followed by the name of the corresponding carboxylic acid in which the suffix ‘ic acid’ is replaced with ‘ate’. For unsaturated carboxylic acid, molecule containing a carbon-carbon double bond or a carbon-carbon triple bond, infix ‘-en-’ and ‘-yn-’ should be used respectively.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: carboxylic amides
Carboxylic amides are organic compounds having R–(C=O)-NH2 or -RCONH2 as their functional groups, where R is alkyl group. The name of carboxylic amides is derived from the name of the corresponding carboxylic acid in which the suffix ‘oic acid’ is replaced with ‘amide’. For unsaturated carboxylic acid, molecule containing a carbon-carbon double bond or a carbon-carbon triple bond, infix ‘-en-’ and ‘-yn-’ should be used respectively. If the nitrogen atom of the amide is bonded to an alkyl or aryl group, it is indicated by prefix N- in the naming.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Naming Organic Compounds: carboxylic anhydrides
Carboxylic anhydrides are organic compounds having R–(C=O)-O-(C=O)R’ or -RCOOCOR’ as their functional groups, where R and R’ are alkyl groups. The name of the carboxylic anhydride is derived from the name of the corresponding carboxylic acid in which the suffix ‘acid’ is replaced with ‘anhydride’ in the parent compound.
The Secret to Naming Organic Compounds in Less Than Ten Minutes
Order of precedence in the functional groups for naming organic compounds,
carboxyl (-oic acid) > aldehyde (-al) > ketone (-one) > alcohol (-ol) > amino (-amine) > sulfhydryl (-thiol)













No comments:
Post a Comment
Please do not enter any spam link in the comment box!